
Quantum Efficiency Tester
PL/EL Integrated System
PV-Reflectumeter
3D Confocal Microscope
In-Line Four Point Probe Tester
Four Point Probe Tester
In-Line Thin Film Thickness Tester
Raman Spectrometer
FTIR Spectrometer
Spectrophotometer
Automatic Spectroscopic Ellipsometer
Contact Resistance Tester
Ultra depth of field 3D microscope
Auto Visual Tester
VMM PV Vision Measuring Machine
Solar Cell Horizontal Tensile Tester
Steady State Solar Simulator for Solar Cell
Solar Cell UV Aging Test Chamber
Solar Cell Comprehensive Tensile Tester
Visual Inspection Tester
Wet Leakage Current Tester
PV Module EL Tester
PV Module UV Preconditioning Chamber
Steady State Solar Simulator for PV Module
Current Continuous Monitor
Potential Induced Degradation Test
Bypass Diode Tester
LeTID Test System
Reverse Current Overload Tester
Impulse Voltage Tester
Hipot Insulation Tester
Ground Continuity Tester
Hipot Insulation Ground Tester
Damp Heat Test Chamber
Humidity Freeze Test
Thermal Cycle Test Chamber
Dynamic Mechanical Load Tester
Static Mechanical Load Tester
Hail Impact Tester
Robustness of Termination Tester
Module Breakage Tester
Cut Susceptibility Tester
Peel Shear Strength Tester
Universal Testing Machine (Single-arm)
Universal Testing Machine (Double-arm)
Glass Transmittance Tester
Acetic Acid Test Chamber
EVA Degree of Crosslinking Test System
Junction Box Comprehensive Tester
Drop ball tester
Semi-automatic scanning four-probe tester
Stylus Profilometer
Maximum Power Point Tracker
Perovskite Glass Transmittance Tester
Perovskite P1 Laser Scribing Multifunctional Testing Machine
Perovskite Online PL Tester
Perovskite Online Sheet Resistance Tester
Online Perovskite Film Thickness Tester
Perovskite Process Inspection Workstation
Portable IV Curve Tester
Portable EL Tester
Portable Thermal Imaging Tester
Solar Module Multi-Channel Testing System
PV Inverter Power Quality Tester
Drone EL Tester
IV Tester
IVEL Cell Sorting Machine
Performance Analysis of BSG and PSG Films
Date : 23 January 2026Views : 80
In the pursuit of higher conversion efficiency in crystalline silicon solar cell technology, borosilicate glass (BSG) and phosphosilicate glass (PSG)—byproducts formed after the diffusion process—have evolved from mere “glass layers requiring removal” into one of the key functional films determining cell performance. Optimizing their properties fundamentally involves a scientific and engineering endeavor to precisely control doping, passivation, optical characteristics, and material properties at the atomic scale.
I. Origin and Fundamental Physicochemical Properties of BSG and PSG
BSG and PSG are not pre-deposited layers. Instead, they form during high-temperature (800–900°C) phosphorus or boron diffusion processes. These layers emerge as boron- or phosphorus-rich silicon dioxide (SiO₂) films when dopant sources (e.g., POCL₃ or BCL₃) react with silicon atoms on the wafer surface. This reaction simultaneously creates P-N junctions or P+ backfields.
PSG (Phosphorus-Silicon Glass): Chemical formula SiO₂:P₂O₅. P₂O₅ forms a phosphoric acid-silicon glass network with SiO₂, essentially involving partial substitution of Si⁴⁺ sites by P⁵⁺ ions, altering the glass network structure.
BSG (Boron-Silicon Glass): Chemical formula SiO₂:B₂O₃. B³⁺ ions also enter the SiO₂ network, but due to differences in valence and ionic radius, their impact on the network structure differs significantly from phosphorus. This often results in more structural defects and instability. Furthermore, B₂O₃'s strong hygroscopicity readily introduces hydroxyl (-OH) groups, which can cause microbubbles or delamination during subsequent high-temperature processes, compromising coating uniformity and interface quality.
The fundamental difference between these two glass layers stems from the solubility and behavior of boron and phosphorus within the SiO₂ network. Phosphorus exhibits high solid solubility in SiO₂, enabling the formation of a stable PSG layer. In contrast, boron has lower solubility, and B₂O₃ readily reacts with water vapor at high temperatures to form volatile compounds. This results in BSG layers that are typically thinner and more non-uniform.
In contrast, PSG not only exhibits a dense structure and high stability, but its phosphorus-rich nature also releases active phosphorus atoms during annealing, enabling self-aligned doping and reduced interface state density. This intrinsic difference dictates their divergence in passivation mechanisms and process compatibility: PSG facilitates high-quality chemical passivation and ohmic contact more readily, whereas BSG requires supplementary hydrogen sources or composite passivation strategies to compensate for its limited chemical passivation capability.
II. Core Performance Optimization Contradictions of BSG/PSG
In conventional processes, borosilicate glass (BSG) and phosphosilicate glass (PSG) are typically removed. However, in selective emitters (SE) and advanced structures like PERC and TOPCon, they are selectively retained or modified to serve dual roles, presenting core performance optimization contradictions.
1. Performance Optimization of PSG
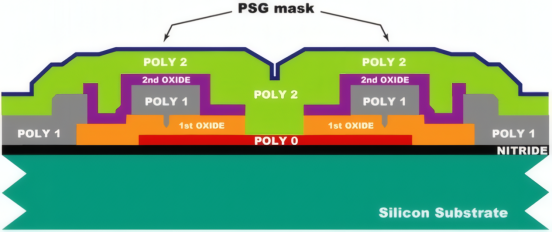
PSG (Phosphorus-Silicon Glass)
The superior passivation of PSG on n-type emitter surfaces primarily stems from field-effect passivation. Its abundant positive charges (derived from P⁵⁺) repel majority carriers (electrons) on the n-type silicon surface, forming a surface electric field that significantly suppresses minority carrier (hole) recombination.
Optimization hinges on balancing:
Phosphorus concentration and distribution: High surface phosphorus concentration enhances the field effect, but excessive levels create a high-recombination “dead layer.” The optimization goal is to create a high-concentration, ultra-thin layer on the surface to enhance the field effect, while maintaining a low-recombination emitter beneath. This is often achieved through laser-selective emitter doping (LDSE): laser-localized processing of PSG creates a heavily doped contact region beneath the electrode, while non-contact areas retain light doping and an intact PSG passivation layer.
Hydrogen Passivation: PSG serves as an excellent hydrogen source. During subsequent hydrogen treatment, hydrogen is released from PSG and diffuses to the silicon interface, passivating defects. Optimizing PSG's density and hydrogen content is crucial.
Impurity Absorption: During high-temperature processes, PSG—as a region with high defect density—attracts and traps metallic impurities (e.g., Fe, Cu) within the silicon body, enhancing the quality of the bulk material. A balance must be achieved between impurity absorption efficiency (requiring thicker, more porous PSG) and optical loss/process complexity. This is typically accomplished by designing dedicated impurity absorption annealing steps.
2. Performance Optimization of BSG
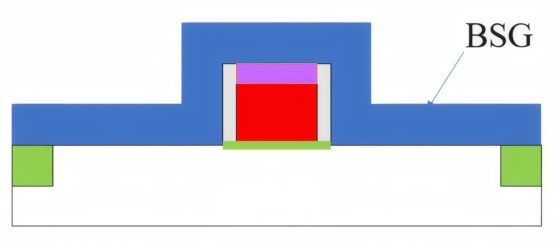
BSG (borosilicate glass)
The passivation mechanism of BSG on p-type silicon primarily relies on chemical passivation, utilizing its network structure or introduced hydrogen to passivate interface states. However, its passivation quality typically falls far short of dedicated dielectric films like Al₂O₃.
Optimization faces dual challenges:
Enhancing Passivation Performance:
• Optimizing boron diffusion processes to achieve a flatter, defect-reduced BSG/Si interface is fundamental.
• A more effective approach involves removing BSG and depositing specialized passivation films like Al₂O₃, leveraging their negative charge trapping effect to achieve superior passivation.
• Simultaneously, optimizing BSG's properties as a hydrogen channel to enhance hydrogen transport toward the p+ backfield interface is crucial for boosting PERC cell voltage (Voc).
Back Contact Formation: During the sintering process of aluminum paste on the battery's rear surface, the retention state of the BSG directly impacts contact quality.
• Excessively thick or uneven BSG hinders the aluminum-silicon eutectic reaction, leading to increased contact resistance.
• The ideal scenario involves forming an ultra-thin, uniform BSG layer that allows easy “penetration” or reaction with the aluminum paste during sintering.
• Therefore, precise control of BSG retention is essential: partial preservation through methods like wet etching allows it to serve as a passivation/hydrogen source while not hindering the formation of good ohmic contact.
Optimization of BSG/PSG cannot be isolated; it must be considered holistically within the entire cell process chain. In TOPCon cells, the traditional heavily doped emitter is replaced by a tunnel oxide layer and polysilicon layer, diminishing PSG's role. In HJT cells, all high-temperature processes are avoided, eliminating BSG/PSG entirely. This signifies a profound shift in cell technology—from “utilizing byproducts” to “designing functional layers.”

































































